
Lungpacer Medical Accelerates Pivotal Clinical Study with AeroPace™ System
Lungpacer Medical, a leading medical device innovation company, today announced the introduction of the AeroPace™ System, a next generation product, into the RESCUE 3 pivotal clinical study studying faster ventilator independence. Lungpacer is dedicated to natural breathing by developing minimally invasive technologies designed to help patients wean off mechanical ventilation and breathe on their own. The RESCUE 3 study is the third in a series of studies to support FDA and international regulatory approvals.

Lungpacer Medical Wins TCT 2021 Shark Tank Innovation Competition
Award recognizes medical device company pioneering a therapy to build diaphragm strength and empower natural, independent breathing for patients who
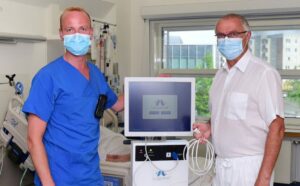
First COVID-19 Patient in Germany successfully treated with novel Diaphragm Therapy
Greifswald University Hospital tests new method (Lungpacer® System) for weaning of respiratory patients Constanze Steinke Pressearbeit Universität Greifswald 07/10/2020 08:54

Lungpacer Medical, Inc. Announces FDA Approval of Emergency Use Authorization of the Lungpacer Diaphragm Pacing Therapy System to Help Address COVID-19 Pandemic
VANCOUVER, British Columbia, April 14, 2020 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced

Lungpacer Medical, Inc. Presentations at American Thoracic Society Conference, Scheduled for May 2020, Philadelphia, Pennsylvania
An average of 14,000 pulmonary, critical care and sleep medicine clinicians and researchers from around the world attend the ATS

Lungpacer Medical, Inc. Presents Preclinical Results at Society of Critical Care Medicine, February 2020, Orlando, Florida
The Society of Critical Care Medicine (SCCM) is the largest non-profit medical organization dedicated to promoting excellence and consistency in

Lungpacer Medical, Inc. Presents Preclinical Results at the European Respiratory Society’s Respiratory Failure & Mechanical Ventilation Conference, February 2020, Berlin, Germany
The inaugural ERS Respiratory Failure and Mechanical Ventilation conference gathered more than 400 delegates from 60 countries, representing a diverse

Lungpacer Medical, Inc. Announces Completion of RESCUE 2 European Clinical Study
VANCOUVER, British Columbia, January 2020 Lungpacer Medical, Inc., announced the completion of enrollment and follow up in its RESCUE 2

Lungpacer Medical, Inc. Announces Publication of RESCUE 2 Study Design Manuscript
VANCOUVER, British Columbia, December 2019 Lungpacer Medical, Inc. announced the publication of the RESCUE 2 Study Design Manuscript in the

Lungpacer Medical, Inc. Presents Preclinical Results at American Association for Respiratory Care Congress, November 2019, New Orleans, Louisiana
Findings from ongoing preclinical research using Lungpacer’s novel phrenic nerve pacing technology was presented at the American Association for Respiratory
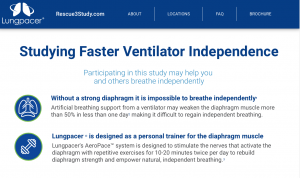
Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 3 Clinical Study
VANCOUVER, British Columbia, June 14, 2019 Lungpacer Medical, Inc., announced today the enrollment of the first subject in the RESCUE

RESCUE 1 Study Findings Presented at American Thoracic Society Conference, May 2019, Dallas, Texas
VANCOUVER, British Columbia, May 2019 Lungpacer Medical, Inc. announced that two abstracts were presented by colleagues at the University of

Lungpacer Medical, Inc. Presents Preclinical Results at American Thoracic Society Conference, May 2019, Dallas, Texas
The ongoing preclinical research using Lungpacer’s novel phrenic nerve pacing technology was well represented at the 2019 American Thoracic Society

Lungpacer Medical, Inc. Presents at Respiratory Innovation Summit, May 2019, Dallas, Texas
Lungpacer Medical, Inc., CEO Doug Evans presented preclinical and clinical research on Lungpacer’s novel phrenic nerve pacing technology at the

Lungpacer Medical, Inc. Announces FDA Approval to Start RESCUE 3 Clinical Study
VANCOUVER, British Columbia, February 22, 2019 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced

Lungpacer Medical, Inc. Presents at The 2018 MedTech Conference, September 2018, Philadelphia, Pennsylvania
Doug Evans, CEO of Lungpacer Medical, Inc., presented at The MedTech Conference in Philadelphia, PA. Sponsored by AdvaMed, The MedTech

Lungpacer Medical, Inc. Presents at WSGR 2018 Medical Device Conference, June 2018, San Francisco, California
Doug Evans, CEO of Lungpacer Medical, Inc., presented at the Wilson Sonsini Goodrich & Rosati 2018 Medical Conference in San

Lungpacer Medical, Inc. Selected as 2018 MedTech Innovator
BURNABY, British Columbia, June 2018 Lungpacer Medical, Inc. was selected out of over 700 applicants to participate in the 2018

ATSJournals Publication – Mitigation of Ventilator-Induced Diaphragm Atrophy by Transvenous Phrenic Nerve
The top-ranked American Journal of Respiratory and Critical Care Medicine has published on-line a pre-clinical research study demonstrating that the Lungpacer therapy mitigated ventilator-induced diaphragm atrophy in pigs that were sedated and ventilated in ICU-like conditions for 60 hours.

Lungpacer Medical, Inc. Announces Completion of Jugular Access Early Feasibility Study
BURNABY, British Columbia, May 2018 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Lungpacer Medical, Inc. Presents Preclinical Data at American Thoracic Society Conference, May 2018, San Francisco, California
This multidisciplinary conference attracts pulmonary and critical care clinicians from around the world. Liz Rohrs presented her preclinical work, which

Lungpacer Medical, Inc. Presents at Respiratory Innovation Summit, May 2018, San Francisco, California
Lungpacer Medical Inc. was invited to present at the inaugural RIS meeting in San Francisco, CA. The Respiratory Innovation Summit

Lungpacer Medical, Inc. Announces Completion of RESCUE 1 Feasibility Study
BURNABY, British Columbia, January 2018 Lungpacer Medical, Inc., announced the completion of enrollment into the RESCUE 1 feasibility study. This

Lungpacer Medical, Inc. Announces the Opening of US Headquarters
BURNABY, British Columbia, January 2018 Lungpacer Medical, Inc. has opened its US headquarters in Exton, PA, with the corporate headquarters

Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 2 European Clinical Study
BURNABY, British Columbia, 29 September 2017 Lungpacer Medical, Inc., announced today that the first subject has been enrolled in the

Lungpacer Medical, Inc. Announces Publication of First-In-Human (FIH) Study Results in Critical Care Medicine
BURNABY, British Columbia, July 2017 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 1 Feasibility Study
BURNABY, British Columbia, 20 June 2017 Lungpacer Medical, Inc., announced today that the first subject has been enrolled in the

Lungpacer Medical, Inc. Announces Publication of Preclinical Findings in American Journal of Respiratory and Critical Care Medicine
BURNABY, British Columbia, February 2017 Lungpacer Medical, Inc., announced today that the findings from preclinical work have been published in

Steven Reynolds, MD to Present Data on the PACER Trial for Lungpacer Medical’s Diaphragm Pacing Catheter in Mechanically Ventilated Patients
BURNABY, British Columbia, May 18, 2016 /PRNewswire/ — Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today that Steven Reynolds, MD will be presenting data related to the recently completed Phrenic Activation for Enhanced Respiration (PACER) feasibility trial at the American Thoracic Society Meeting in San Francisco, California on Wednesday, May 18, 2016.

Lungpacer Medical, Inc. Receives Expedited Access Pathway Designation from FDA for the Lungpacer Diaphragm Pacing System
Burnaby, British Columbia, Canada – May 11, 2016 – Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today that it has received Expedited Access Pathway (EAP) designation from the U.S. Food and Drug Administration (FDA).

Lungpacer Medical, Inc. Announces the Completion of First-In-Human Early Feasibility Study
BURNABY, British Columbia, May 2016 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Lungpacer Medical, Inc. Presents at 2016 Innovation Summit, April 2016, Dublin, Ireland
Lungpacer Medical, Inc. was invited to present at the 2016 Innovation Summit in Dublin, Ireland, the leading medical technology investment

Lungpacer Medical, Inc. Announces Start of First-in-Human Early Feasibility Trial
BURNABY, British Columbia, Oct. 21, 2015 /PRNewswire/ — Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today the enrollment of the first patients in the Phrenic ACtivation for Enhanced Respiration (PACER) early feasibility trial.

Lungpacer Medical, Inc. to Present at Jefferies 2015 Global Healthcare Conference
Lungpacer Medical, Inc. to Present at Jefferies 2015 Global Healthcare Conference

Lungpacer named a “2015 Ready to Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

Lungpacer expands into new location
SFU spinout Lungpacer Medical Inc. welcomed nearly 100 guests on Dec. 11 to its new 6,500-sq.-ft. Burnaby premises, which are 3 1/2

CIHR Proof-of-Principle Grant for First-in-Human Trials at Royal Columbian Hospital
Dr. Steve Reynolds, head of Critical Care and infectious diseases specialist at Royal Columbian Hospital, and Dr. Andy Hoffer, founder of Lungpacer Medical Inc., are collaborating to improve how intensive care units provide respiratory support for their sickest patients.

Lungpacer named a “2013 Emerging Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

Lungpacer wins Silver Award at World’s Best Technologies Innovation Showcase, San Diego
Lungpacer Medical Inc. takes silver for its Lungpacer Diaphragm Pacing System at the World’s Best Technology Showcase (WBTS), San Diego on October 26, 2012.

Lungpacer wins BCTIA 2012 Most Promising Pre-Commercial Technology Award
Lungpacer Medical Inc., has won the BC Technology Industry Association’s 2012 award for the Most Promising Pre-Commercial Technology.

Lungpacer showcased by RHI at Interdependence 2012 Global SCI Conference
Lungpacer showcased by RHI at Interdependence 2012 Global SCI Conference

Lungpacer a BCTIA Most Promising Pre-Commercial Technology 2012 finalist
VANCOUVER, BC, May 2, 2012 – Today the BC Technology Industry Association (BCTIA) announced the finalists for the 2012 Technology Impact Awards (TIAs).

Lungpacer named a “2012 Emerging Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

IFESS 2010 Conference Presentation, Vienna, Austria
Full Text: IFESS 2010 Hoffer et al Diaphragm Pacing with Endovascular Electrodes September 2010

NRC IRAP contributes $130,000 to Lungpacer Control Unit development
NRC IRAP contributes $130,000 to Lungpacer Control Unit development

Lungpacer Medical, Inc. Presents Preclinical Results at American Association for Respiratory Care Congress, November 2019, New Orleans, Louisiana
Findings from ongoing preclinical research using Lungpacer’s novel phrenic nerve pacing technology was presented at the American Association for Respiratory

RESCUE 1 Study Findings Presented at American Thoracic Society Conference, May 2019, Dallas, Texas
VANCOUVER, British Columbia, May 2019 Lungpacer Medical, Inc. announced that two abstracts were presented by colleagues at the University of

Lungpacer Medical, Inc. Presents at WSGR 2018 Medical Device Conference, June 2018, San Francisco, California
Doug Evans, CEO of Lungpacer Medical, Inc., presented at the Wilson Sonsini Goodrich & Rosati 2018 Medical Conference in San

ATSJournals Publication – Mitigation of Ventilator-Induced Diaphragm Atrophy by Transvenous Phrenic Nerve
The top-ranked American Journal of Respiratory and Critical Care Medicine has published on-line a pre-clinical research study demonstrating that the Lungpacer therapy mitigated ventilator-induced diaphragm atrophy in pigs that were sedated and ventilated in ICU-like conditions for 60 hours.

Lungpacer Medical, Inc. Presents Preclinical Data at American Thoracic Society Conference, May 2018, San Francisco, California
This multidisciplinary conference attracts pulmonary and critical care clinicians from around the world. Liz Rohrs presented her preclinical work, which

Lungpacer Medical, Inc. Presents at Respiratory Innovation Summit, May 2018, San Francisco, California
Lungpacer Medical Inc. was invited to present at the inaugural RIS meeting in San Francisco, CA. The Respiratory Innovation Summit

Lungpacer Medical, Inc. Announces Completion of RESCUE 1 Feasibility Study
BURNABY, British Columbia, January 2018 Lungpacer Medical, Inc., announced the completion of enrollment into the RESCUE 1 feasibility study. This

Lungpacer Medical, Inc. Announces the Opening of US Headquarters
BURNABY, British Columbia, January 2018 Lungpacer Medical, Inc. has opened its US headquarters in Exton, PA, with the corporate headquarters

Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 2 European Clinical Study
BURNABY, British Columbia, 29 September 2017 Lungpacer Medical, Inc., announced today that the first subject has been enrolled in the

Lungpacer Medical, Inc. Announces Publication of First-In-Human (FIH) Study Results in Critical Care Medicine
BURNABY, British Columbia, July 2017 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 1 Feasibility Study
BURNABY, British Columbia, 20 June 2017 Lungpacer Medical, Inc., announced today that the first subject has been enrolled in the

Lungpacer Medical, Inc. Announces Publication of Preclinical Findings in American Journal of Respiratory and Critical Care Medicine
BURNABY, British Columbia, February 2017 Lungpacer Medical, Inc., announced today that the findings from preclinical work have been published in

Lungpacer Medical, Inc. Receives Expedited Access Pathway Designation from FDA for the Lungpacer Diaphragm Pacing System
Burnaby, British Columbia, Canada – May 11, 2016 – Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today that it has received Expedited Access Pathway (EAP) designation from the U.S. Food and Drug Administration (FDA).

Lungpacer Medical, Inc. Announces the Completion of First-In-Human Early Feasibility Study
BURNABY, British Columbia, May 2016 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Lungpacer Medical, Inc. Presents at 2016 Innovation Summit, April 2016, Dublin, Ireland
Lungpacer Medical, Inc. was invited to present at the 2016 Innovation Summit in Dublin, Ireland, the leading medical technology investment

Lungpacer Medical, Inc. Announces Start of First-in-Human Early Feasibility Trial
BURNABY, British Columbia, Oct. 21, 2015 /PRNewswire/ — Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today the enrollment of the first patients in the Phrenic ACtivation for Enhanced Respiration (PACER) early feasibility trial.

Lungpacer named a “2015 Ready to Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

NRC IRAP contributes $130,000 to Lungpacer Control Unit development
NRC IRAP contributes $130,000 to Lungpacer Control Unit development

Lungpacer Medical Accelerates Pivotal Clinical Study with AeroPace™ System
Lungpacer Medical, a leading medical device innovation company, today announced the introduction of the AeroPace™ System, a next generation product, into the RESCUE 3 pivotal clinical study studying faster ventilator independence. Lungpacer is dedicated to natural breathing by developing minimally invasive technologies designed to help patients wean off mechanical ventilation and breathe on their own. The RESCUE 3 study is the third in a series of studies to support FDA and international regulatory approvals.

Lungpacer Medical Wins TCT 2021 Shark Tank Innovation Competition
Award recognizes medical device company pioneering a therapy to build diaphragm strength and empower natural, independent breathing for patients who

First COVID-19 Patient in Germany successfully treated with novel Diaphragm Therapy
Greifswald University Hospital tests new method (Lungpacer® System) for weaning of respiratory patients Constanze Steinke Pressearbeit Universität Greifswald 07/10/2020 08:54

Lungpacer Medical, Inc. Announces FDA Approval of Emergency Use Authorization of the Lungpacer Diaphragm Pacing Therapy System to Help Address COVID-19 Pandemic
VANCOUVER, British Columbia, April 14, 2020 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced

Lungpacer Medical, Inc. Presentations at American Thoracic Society Conference, Scheduled for May 2020, Philadelphia, Pennsylvania
An average of 14,000 pulmonary, critical care and sleep medicine clinicians and researchers from around the world attend the ATS

Lungpacer Medical, Inc. Presents Preclinical Results at Society of Critical Care Medicine, February 2020, Orlando, Florida
The Society of Critical Care Medicine (SCCM) is the largest non-profit medical organization dedicated to promoting excellence and consistency in

Lungpacer Medical, Inc. Presents Preclinical Results at the European Respiratory Society’s Respiratory Failure & Mechanical Ventilation Conference, February 2020, Berlin, Germany
The inaugural ERS Respiratory Failure and Mechanical Ventilation conference gathered more than 400 delegates from 60 countries, representing a diverse

Lungpacer Medical, Inc. Announces Completion of RESCUE 2 European Clinical Study
VANCOUVER, British Columbia, January 2020 Lungpacer Medical, Inc., announced the completion of enrollment and follow up in its RESCUE 2

Lungpacer Medical, Inc. Announces Publication of RESCUE 2 Study Design Manuscript
VANCOUVER, British Columbia, December 2019 Lungpacer Medical, Inc. announced the publication of the RESCUE 2 Study Design Manuscript in the

Lungpacer Medical, Inc. Announces Enrollment of First Subject in RESCUE 3 Clinical Study
VANCOUVER, British Columbia, June 14, 2019 Lungpacer Medical, Inc., announced today the enrollment of the first subject in the RESCUE

Lungpacer Medical, Inc. Presents Preclinical Results at American Thoracic Society Conference, May 2019, Dallas, Texas
The ongoing preclinical research using Lungpacer’s novel phrenic nerve pacing technology was well represented at the 2019 American Thoracic Society

Lungpacer Medical, Inc. Presents at Respiratory Innovation Summit, May 2019, Dallas, Texas
Lungpacer Medical, Inc., CEO Doug Evans presented preclinical and clinical research on Lungpacer’s novel phrenic nerve pacing technology at the

Lungpacer Medical, Inc. Announces FDA Approval to Start RESCUE 3 Clinical Study
VANCOUVER, British Columbia, February 22, 2019 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced

Lungpacer Medical, Inc. Presents at The 2018 MedTech Conference, September 2018, Philadelphia, Pennsylvania
Doug Evans, CEO of Lungpacer Medical, Inc., presented at The MedTech Conference in Philadelphia, PA. Sponsored by AdvaMed, The MedTech

Lungpacer Medical, Inc. Selected as 2018 MedTech Innovator
BURNABY, British Columbia, June 2018 Lungpacer Medical, Inc. was selected out of over 700 applicants to participate in the 2018

Lungpacer Medical, Inc. Announces Completion of Jugular Access Early Feasibility Study
BURNABY, British Columbia, May 2018 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today

Steven Reynolds, MD to Present Data on the PACER Trial for Lungpacer Medical’s Diaphragm Pacing Catheter in Mechanically Ventilated Patients
BURNABY, British Columbia, May 18, 2016 /PRNewswire/ — Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing system, announced today that Steven Reynolds, MD will be presenting data related to the recently completed Phrenic Activation for Enhanced Respiration (PACER) feasibility trial at the American Thoracic Society Meeting in San Francisco, California on Wednesday, May 18, 2016.

Lungpacer Medical, Inc. to Present at Jefferies 2015 Global Healthcare Conference
Lungpacer Medical, Inc. to Present at Jefferies 2015 Global Healthcare Conference

Lungpacer expands into new location
SFU spinout Lungpacer Medical Inc. welcomed nearly 100 guests on Dec. 11 to its new 6,500-sq.-ft. Burnaby premises, which are 3 1/2

CIHR Proof-of-Principle Grant for First-in-Human Trials at Royal Columbian Hospital
Dr. Steve Reynolds, head of Critical Care and infectious diseases specialist at Royal Columbian Hospital, and Dr. Andy Hoffer, founder of Lungpacer Medical Inc., are collaborating to improve how intensive care units provide respiratory support for their sickest patients.

Lungpacer named a “2013 Emerging Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

Lungpacer wins Silver Award at World’s Best Technologies Innovation Showcase, San Diego
Lungpacer Medical Inc. takes silver for its Lungpacer Diaphragm Pacing System at the World’s Best Technology Showcase (WBTS), San Diego on October 26, 2012.

Lungpacer wins BCTIA 2012 Most Promising Pre-Commercial Technology Award
Lungpacer Medical Inc., has won the BC Technology Industry Association’s 2012 award for the Most Promising Pre-Commercial Technology.

Lungpacer showcased by RHI at Interdependence 2012 Global SCI Conference
Lungpacer showcased by RHI at Interdependence 2012 Global SCI Conference

Lungpacer a BCTIA Most Promising Pre-Commercial Technology 2012 finalist
VANCOUVER, BC, May 2, 2012 – Today the BC Technology Industry Association (BCTIA) announced the finalists for the 2012 Technology Impact Awards (TIAs).

Lungpacer named a “2012 Emerging Rocket Life Science Company”
Ready to Rocket is a unique business recognition list that profiles British Columbia technology companies with the greatest potential for revenue growth.

IFESS 2010 Conference Presentation, Vienna, Austria
Full Text: IFESS 2010 Hoffer et al Diaphragm Pacing with Endovascular Electrodes September 2010
