Lungpacer
AeroPace™ System
Designed as a personal trainer
for the diaphragm
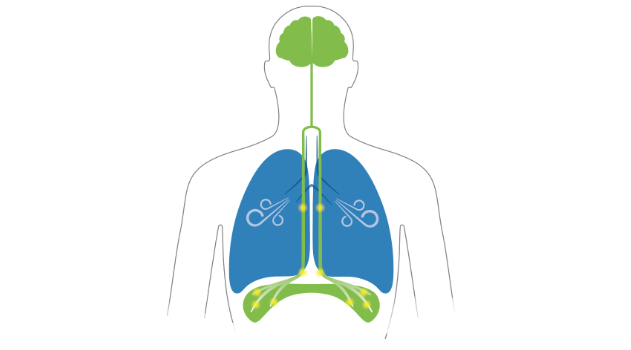
AeroPace system uses the body’s nervous system to send messages to the diaphragm muscle to the source of natural breathing. For patients unable to breathe on their own, AeroPace therapy provides repetitive exercises aiming to rebuild diaphragm muscle strength critical to healthy breathing.
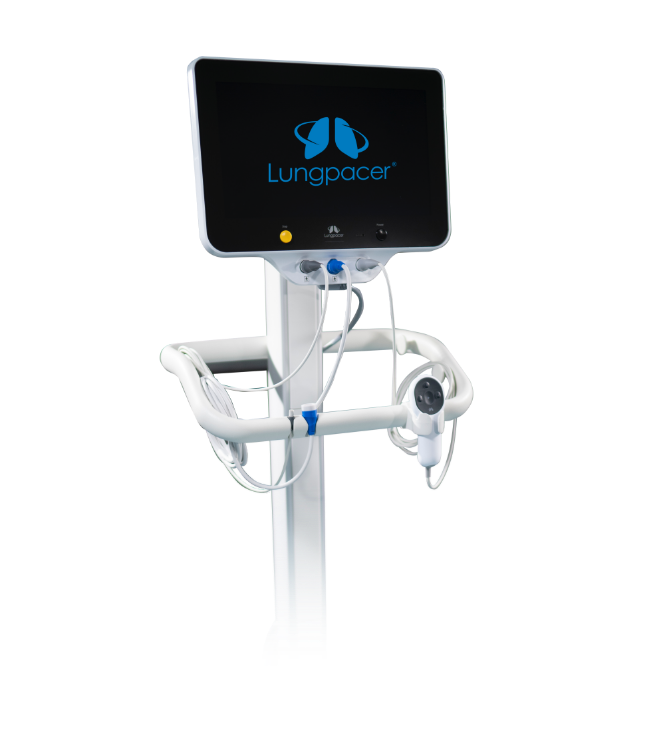
AeroPace™ Controller
For patients unable to breathe on their own, AeroPace therapy provides repetitive exercises intended to rebuild diaphragm muscle strength critical to healthy breathing so patients may wean off the ventilator faster. The AeroPace neurostimulation console sends a signal to the electrodes on the AeroPace catheter to stimulate the phrenic nerve and activate the diaphragm. Like a personal trainer, AeroPace is used to perform repetitive exercises that activate the diaphragm muscle.
Key features:
- Easy setup for convenient patient therapy
- ECG guided placement of catheter designed to eliminate the need for chest x-ray confirmation
- Automated electrode mapping for customized therapy delivery and patient comfort
- Advanced patient synchrony with stimulation up to every breath
- Variable stimulation settings to adjust exercise intensity
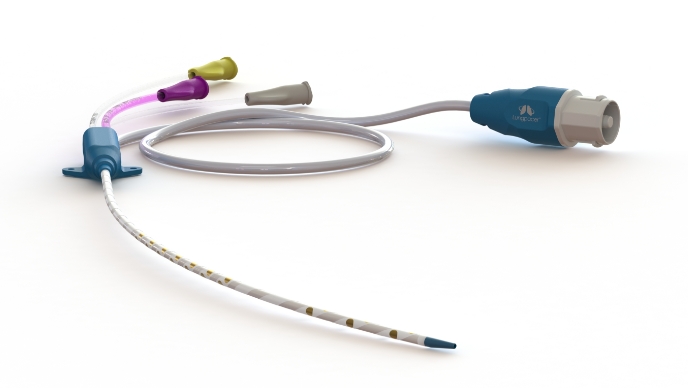
Multi-function AeroPace™ Catheter
Key features:
- Left jugular & left subclavian vein access
- High flow triple lumen central line for medication and fluid delivery
- 8.5 Fr.
- 30 advanced electrodes
- MRI compatible
Frequently Asked Questions
AeroPace therapy is an investigational treatment intended to help strengthen the diaphragm, the large muscle beneath the lungs that plays an important part in breathing. While a person is on a mechanical ventilator (or breathing machine), the diaphragm does not get much use, because the ventilator is doing the breathing. As with all muscles, when it is not used, the diaphragm can lose strength which makes it harder for a person to be weaned from (or no longer need) the ventilator. Lungpacer therapy is designed to stimulate the nerves that activate the diaphragm to keep it in ‘shape’ and may help people wean from the ventilator more quickly to regain independent breathing.
Study risks include those that may occur with the use of central venous catheters and the risks of nerve and diaphragm stimulation. These may include the risks of infection, blood vessel damage, or cardiac effects that can occur with use of catheters. Stimulation risks are rare and may include nerve injury or fatigue of the breathing muscle. Your doctor will discuss these and other risks with you.
Several hundred patients at prominent research institutions in the U.S. and Europe have participated in research in Lungpacer’s technology over the past five years. RESCUE 3 is the pivotal study – the culmination of all the existing research – and the final step needed to seek FDA approval for the AeroPace System.
1. Dres, M., et al. Temporary transvenous diaphragm neurostimulation in difficult-to-wean mechanically ventilated patients – results of the RESCUE 2 randomized controlled trial. Eur Resp J 2020; 56(64): 4352: 246% stronger diaphragm (MIP)/P=0.0010; +7.4% increased ventilator independence/P=0.586; 7.9% greater survival /P=0.216; 1.4 days less dependent on a ventilator/P=0.498. Modified Intent to Treat Subset (mITT).
2. Lungpacer Data on File: 128% improved lung function (RSBI)/P=0.102, not significant. Modified Intent To Treat Subset (mITT).
3. Dres M, Gama De Abreu M, Similowski T. Temporary Transvenous Diaphragm Neurostimulation in Mechanically Ventilated Patients: Per Protocol Results from the RESCUE-2 Randomized Controlled Trial. Am J of Respir Crit Care Med 2021;203: A4668: 167% improved lung function (RSBI)/P=0.0487.
The U.S. Food and Drug Administration (FDA) granted this important designation to Lungpacer’s technology in recognition of its potential to provide more effective treatment than the standard of care available today for patients who have difficulty getting off of mechanical ventilation. The goal of the Breakthrough Devices Program is to provide patients and health care providers with timely access to medical devices like the AeroPace system by prioritizing their development, assessment, and regulatory review consistent with the Agency’s mission to protect and promote public health. More information at https://www.fda.gov/medical-devices/how-study-and-market-your-device/breakthrough-devices-program.
The Lungpacer Medical team is comprised of highly experienced clinical research and engineering teams dedicated to developing technologies to empower independent breathing and free patients from the trauma of mechanical ventilation. Lungpacer Medical is headquartered in Vancouver, Canada with U.S. operations in Exton, Pennsylvania, and team members throughout Europe. More information at www.Lungpacer.com
1. Evans D et al. Temporary transvenous diaphragm pacing vs. standard of care for weaning from mechanical ventilation: study protocol for a randomized trial. Trials. 2019 Jan 17;20(1):60
This is the pivotal clinical study, the third in a series of studies necessary to gain FDA approval for Lungpacer AeroPace system. This is not an early investigational study.
Most people on mechanical ventilation already have a central venous catheter (CVC) inserted into a vein in the upper left chest or neck area. This sterile tube is commonly used to deliver fluids and medications. The multi-function AeroPace catheter delivers fluids and medications as well as electrical stimulations for 10-20 minutes, twice per day to stimulate the nerves which activate and exercise the diaphragm. In many cases, the AeroPace catheter may replace an existing CVC.
The therapy is adjusted to the patient’s comfort. At times, stimulation may be uncomfortable in your breathing muscle, chest, shoulder or abdomen. Those who have received the therapy have described it as feeling like they have the hiccups or a rumbling in their mid-section which stops when the therapy session is done.