
Lungpacer Medical Accelerates Pivotal Clinical Study with AeroPace™ System
Lungpacer Medical, a leading medical device innovation company, today announced the introduction of the AeroPace™ System, a next generation product, into the RESCUE 3 pivotal clinical study studying faster ventilator independence. Lungpacer is dedicated to natural breathing by developing minimally invasive technologies designed to help patients wean off mechanical ventilation and breathe on their own. The RESCUE 3 study is the third in a series of studies to support FDA and international regulatory approvals.

Lungpacer Medical Wins TCT 2021 Shark Tank Innovation Competition
Award recognizes medical device company pioneering a therapy to build diaphragm strength and empower natural, independent breathing for
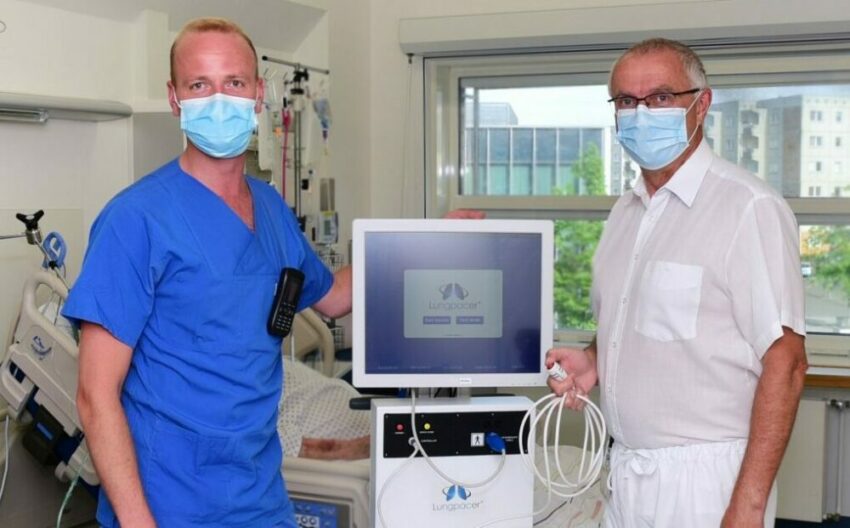
First COVID-19 Patient in Germany successfully treated with novel Diaphragm Therapy
Greifswald University Hospital tests new method (Lungpacer® System) for weaning of respiratory patients Constanze Steinke Pressearbeit Universität Greifswald

Lungpacer Medical, Inc. Announces FDA Approval of Emergency Use Authorization of the Lungpacer Diaphragm Pacing Therapy System to Help Address COVID-19 Pandemic
VANCOUVER, British Columbia, April 14, 2020 Lungpacer Medical, Inc., a medical device company developing an intravenous catheter-based phrenic-nerve-pacing

Lungpacer Medical Accelerates Pivotal Clinical Study with AeroPace™ System
Lungpacer Medical, a leading medical device innovation company, today announced the introduction of the AeroPace™ System, a next generation product, into the RESCUE 3 pivotal clinical study studying faster ventilator independence. Lungpacer is dedicated to natural breathing by developing minimally invasive technologies designed to help patients wean off mechanical ventilation and breathe on their own. The RESCUE 3 study is the third in a series of studies to support FDA and international regulatory approvals.

Lungpacer Medical, Inc. Presents Preclinical Results at Society of Critical Care Medicine, February 2020, Orlando, Florida
The Society of Critical Care Medicine (SCCM) is the largest non-profit medical organization dedicated to promoting excellence and consistency in

Lungpacer Medical, Inc. Presents Preclinical Results at the European Respiratory Society’s Respiratory Failure & Mechanical Ventilation Conference, February 2020, Berlin, Germany
The inaugural ERS Respiratory Failure and Mechanical Ventilation conference gathered more than 400 delegates from 60 countries, representing a diverse

Lungpacer Medical, Inc. Announces Completion of RESCUE 2 European Clinical Study
VANCOUVER, British Columbia, January 2020 Lungpacer Medical, Inc., announced the completion of enrollment and follow up in its RESCUE 2

Lungpacer Medical, Inc. Announces Publication of RESCUE 2 Study Design Manuscript
VANCOUVER, British Columbia, December 2019 Lungpacer Medical, Inc. announced the publication of the RESCUE 2 Study Design Manuscript in the

Lungpacer Medical, Inc. Presents Preclinical Results at American Association for Respiratory Care Congress, November 2019, New Orleans, Louisiana
Findings from ongoing preclinical research using Lungpacer’s novel phrenic nerve pacing technology was presented at the American Association for Respiratory

RESCUE 1 Study Findings Presented at American Thoracic Society Conference, May 2019, Dallas, Texas
VANCOUVER, British Columbia, May 2019 Lungpacer Medical, Inc. announced that two abstracts were presented by colleagues at the University of

Lungpacer Medical, Inc. Presents at WSGR 2018 Medical Device Conference, June 2018, San Francisco, California
Doug Evans, CEO of Lungpacer Medical, Inc., presented at the Wilson Sonsini Goodrich & Rosati 2018 Medical Conference in San

Lungpacer Medical Accelerates Pivotal Clinical Study with AeroPace™ System
Lungpacer Medical, a leading medical device innovation company, today announced the introduction of the AeroPace™ System, a next generation product, into the RESCUE 3 pivotal clinical study studying faster ventilator independence. Lungpacer is dedicated to natural breathing by developing minimally invasive technologies designed to help patients wean off mechanical ventilation and breathe on their own. The RESCUE 3 study is the third in a series of studies to support FDA and international regulatory approvals.

Lungpacer Medical, Inc. Presents Preclinical Results at Society of Critical Care Medicine, February 2020, Orlando, Florida
The Society of Critical Care Medicine (SCCM) is the largest non-profit medical organization dedicated to promoting excellence and consistency in

Lungpacer Medical, Inc. Presents Preclinical Results at the European Respiratory Society’s Respiratory Failure & Mechanical Ventilation Conference, February 2020, Berlin, Germany
The inaugural ERS Respiratory Failure and Mechanical Ventilation conference gathered more than 400 delegates from 60 countries, representing a diverse
Lungpacer Media
For media inquiries, please email info@lungpacer.com.
