Dedicated to
Natural Breathing
Designed to help patients wean off
a ventilator and breathe on their own


Without a strong diaphragm it is impossible to breathe independently*

Lungpacer is designed as a personal trainer for the diaphragm muscle*
Potential Benefits
Wean off a
ventilator faster*
Minimize
ventilator trauma*
Save
cost and
time*
Greater
chance
of survival*
Better
quality
of life*
Our Vision for the Future of Patient Care
Ventilator
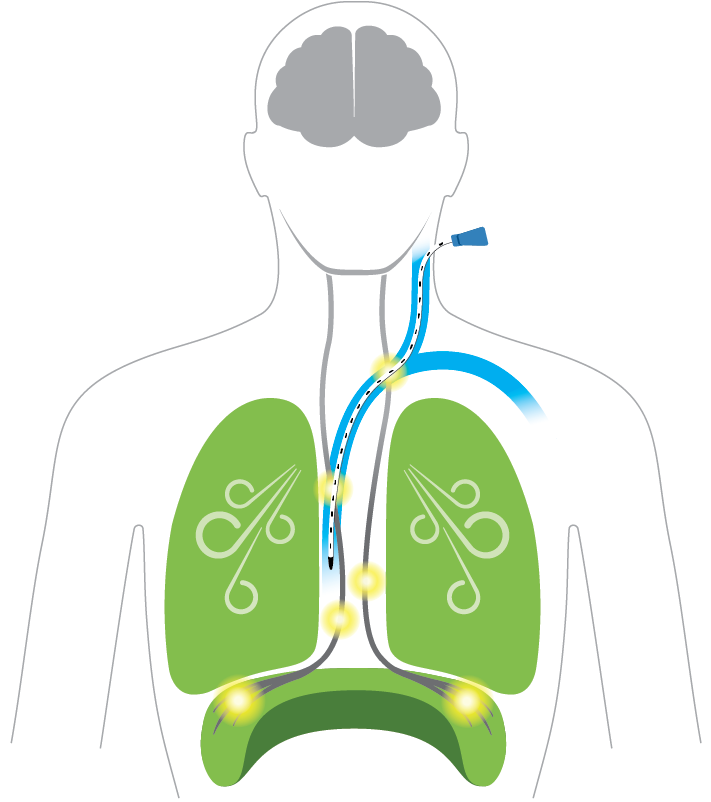
Breathing Complications
Ventilator breathing bypasses the brain and diaphragm by forcing air into the lungs causing damage in less than one day
Lungpacer
Designed to Empower Natural Breathing
Engage the diaphragm, lungs, and brain
to support natural breathing
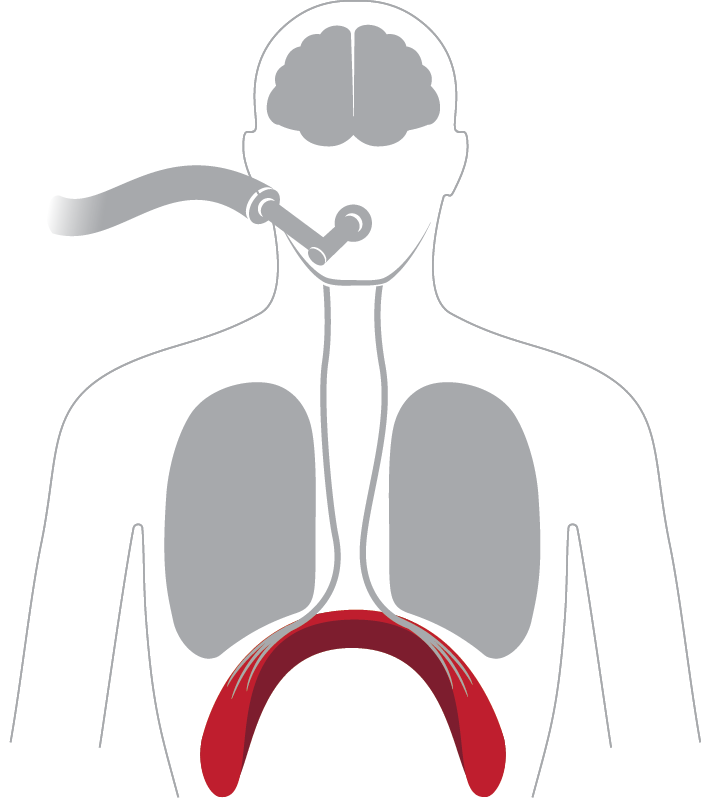
Diaphragm: Inactive
Causes muscle weakness and inability to wean off ventilator, long term complications and higher risk of death*
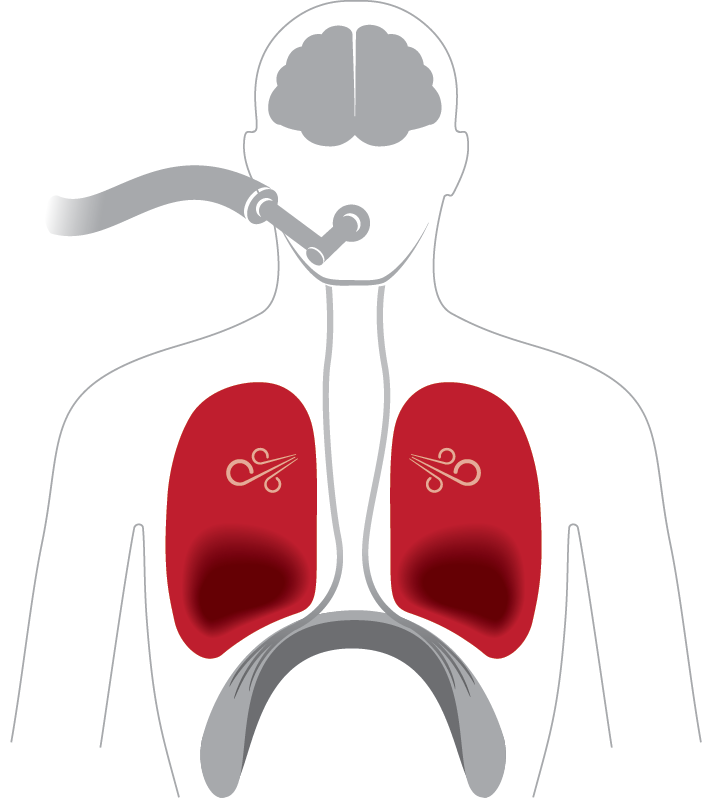
Lungs: Unevenly inflated
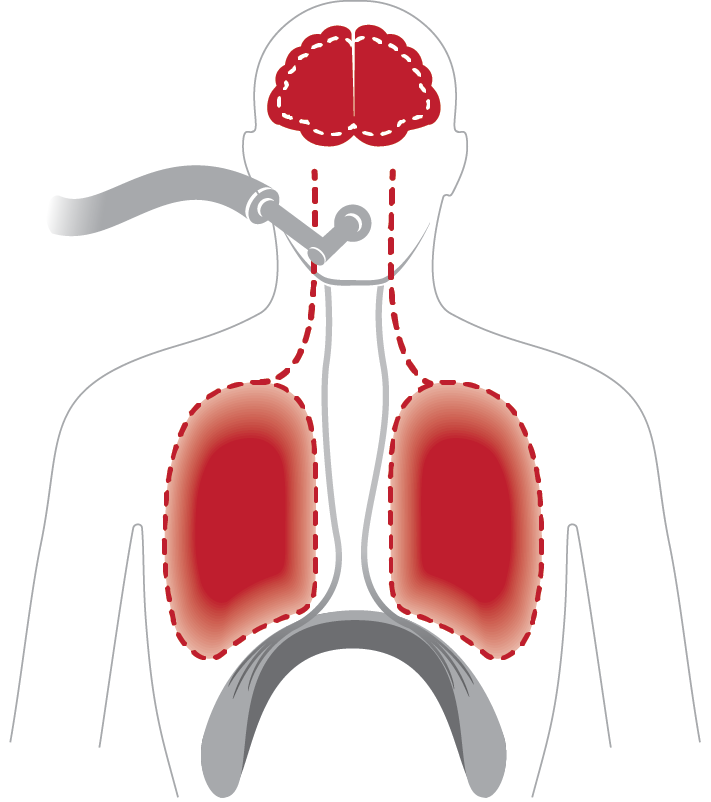
Brain: Signal impaired
Causes cell death and cognitive injury*
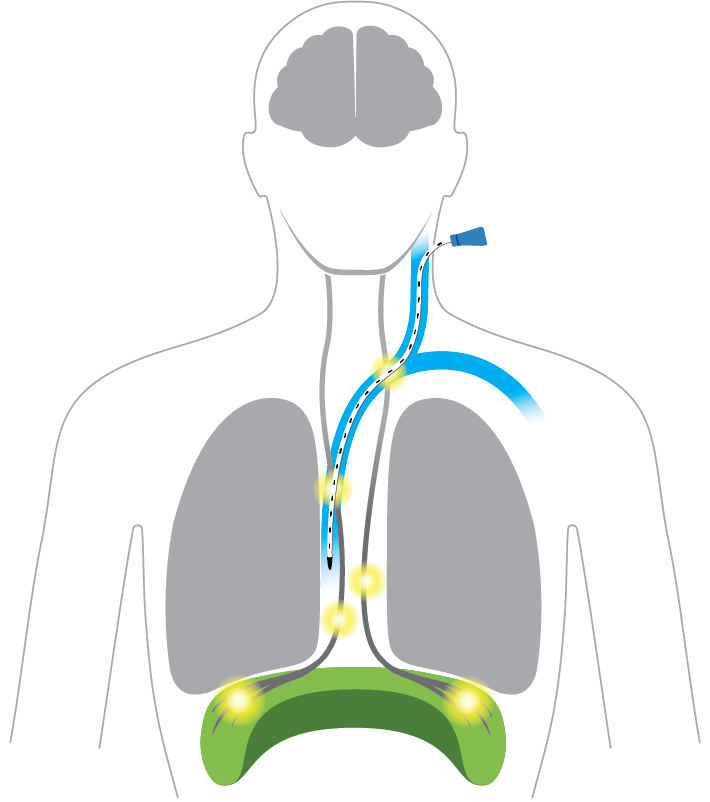
Diaphragm: Exercised
Strengthen muscle through stimulated contractions to accelerate ventilator weaning*
Lungs: Naturally inflated
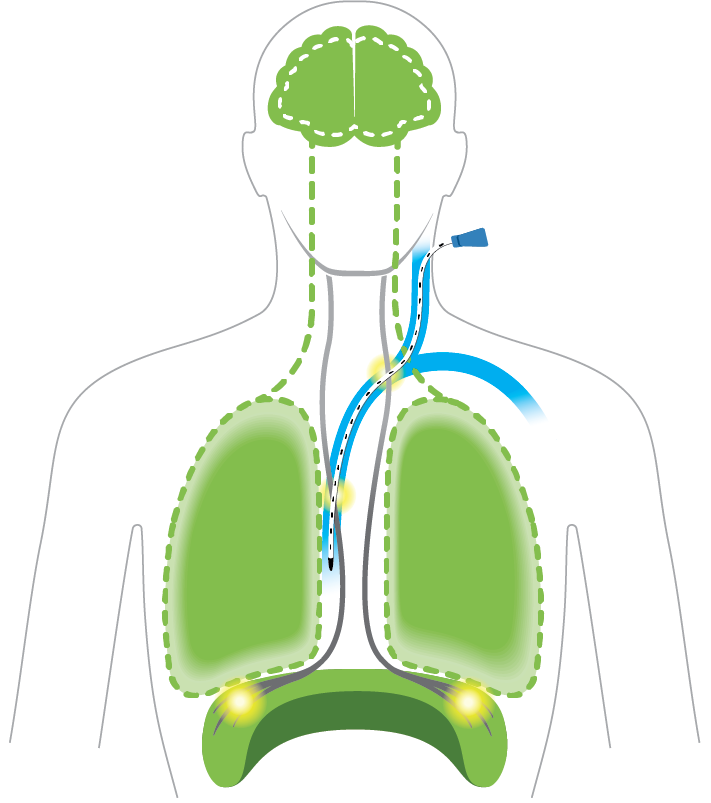
Brain: Signal normalized
Outcomes from a Lungpacer Clinical Study Showed
Lungpacer strengthened the diaphragm and improved lung function
Diaphragm*(MIP)
Lung Function*(RSBI)
Positive trending clinical outcomes
Ventilator Weaning*
Survival*
These results from a randomized, controlled trial compared patients treated with Lungpacer therapy to patients treated with the standard of care. Improvement was statistically significant at p<0.05 for MIP and RSBI; p>0.05 for clinical trends. The rate of serious adverse events was the same between both groups. No unanticipated adverse device effects. Device safety events were those commonly reported for central venous catheters.
Lungpacer Medical Research
& Presentations
Publications
Studied
Learn more about results and trends from more than five years of Lungpacer research.


Lungpacer AeroPace™ System
For patients unable to breathe on their own, the AeroPace therapy is designed to provide repetitive exercises, to rebuild diaphragm muscle strength critical to healthy breathing.
