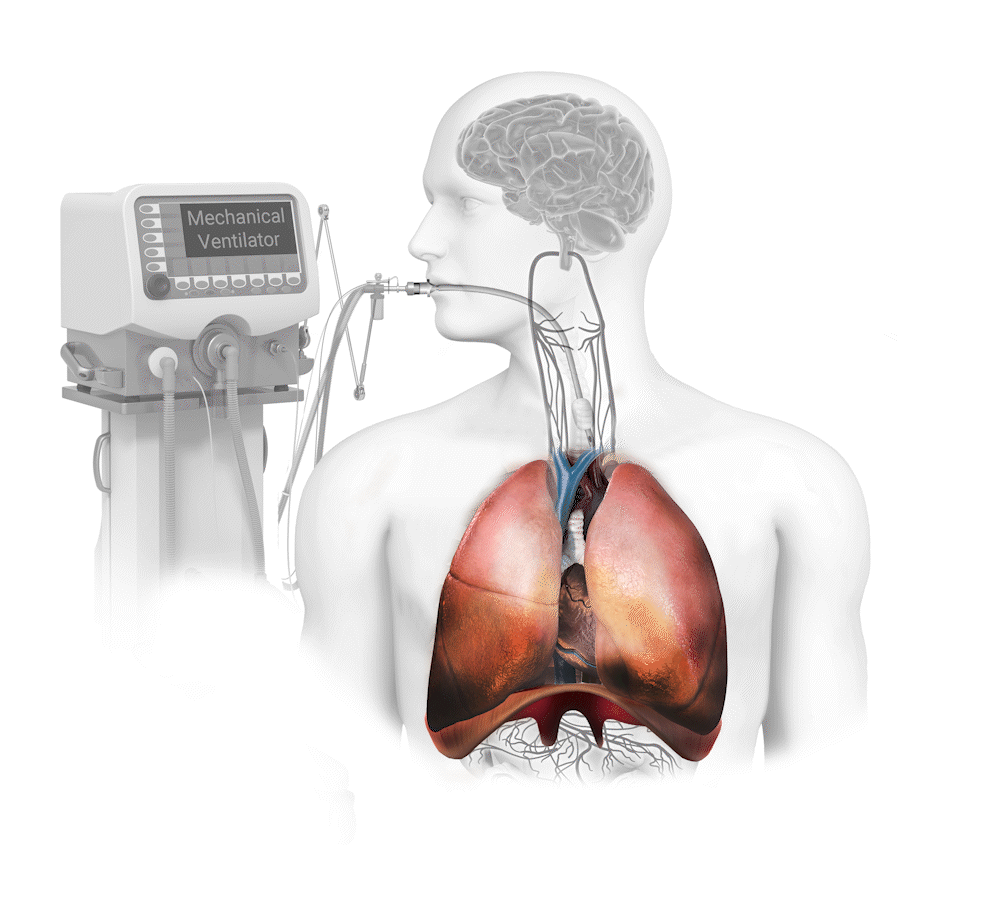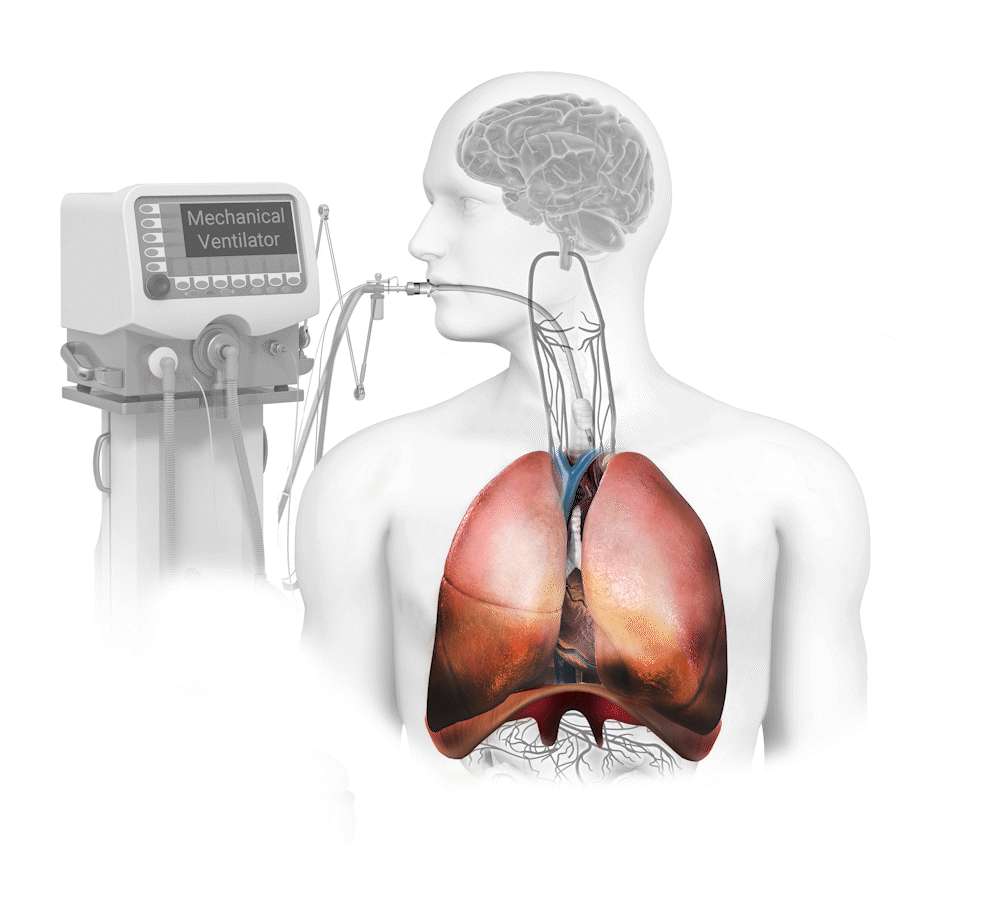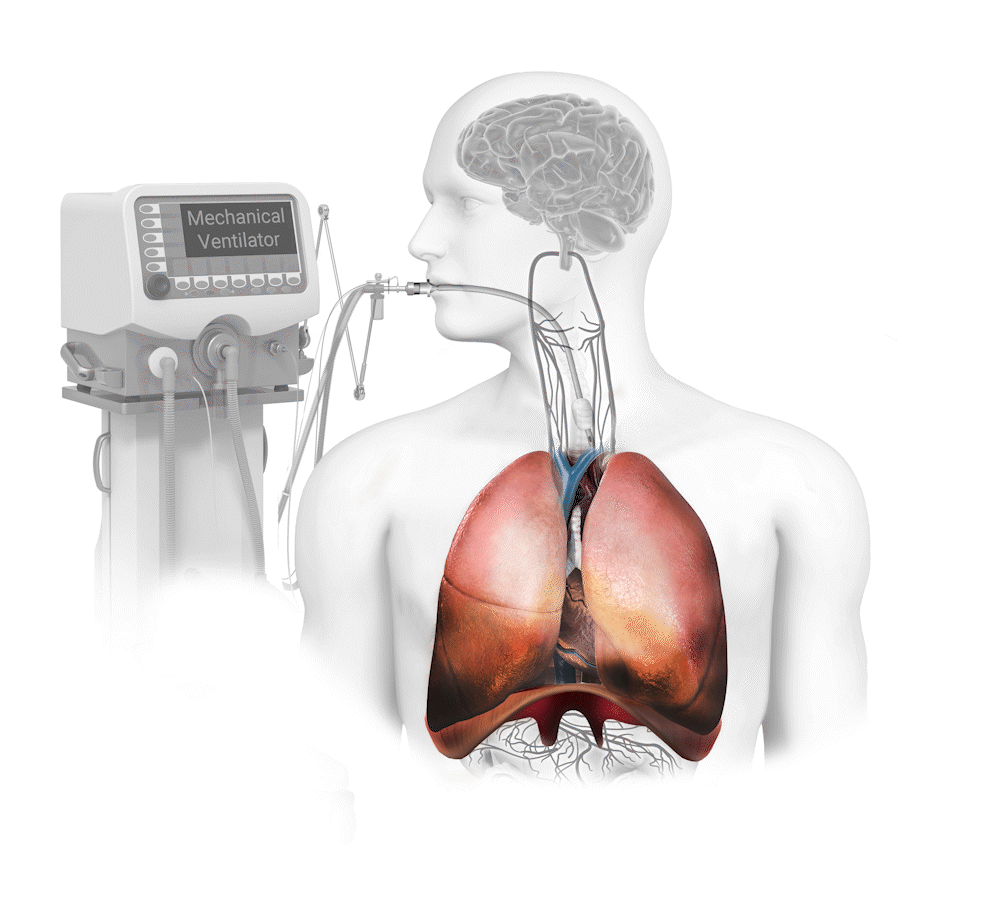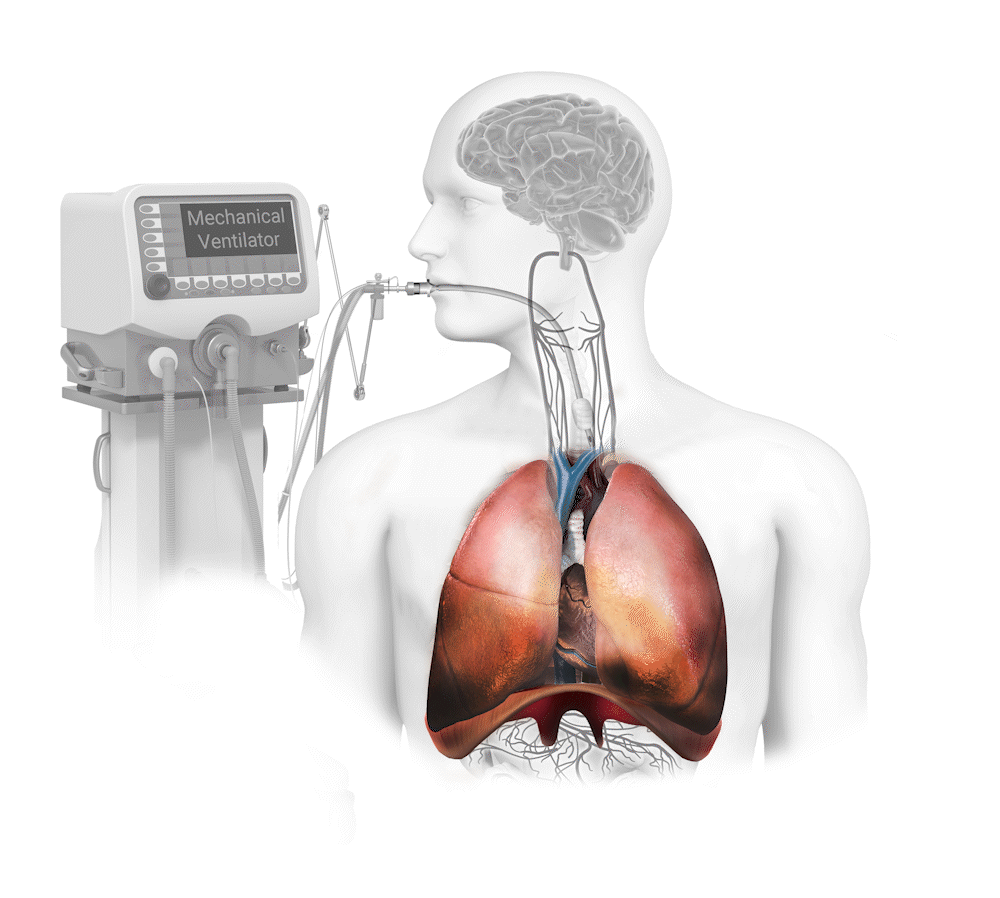
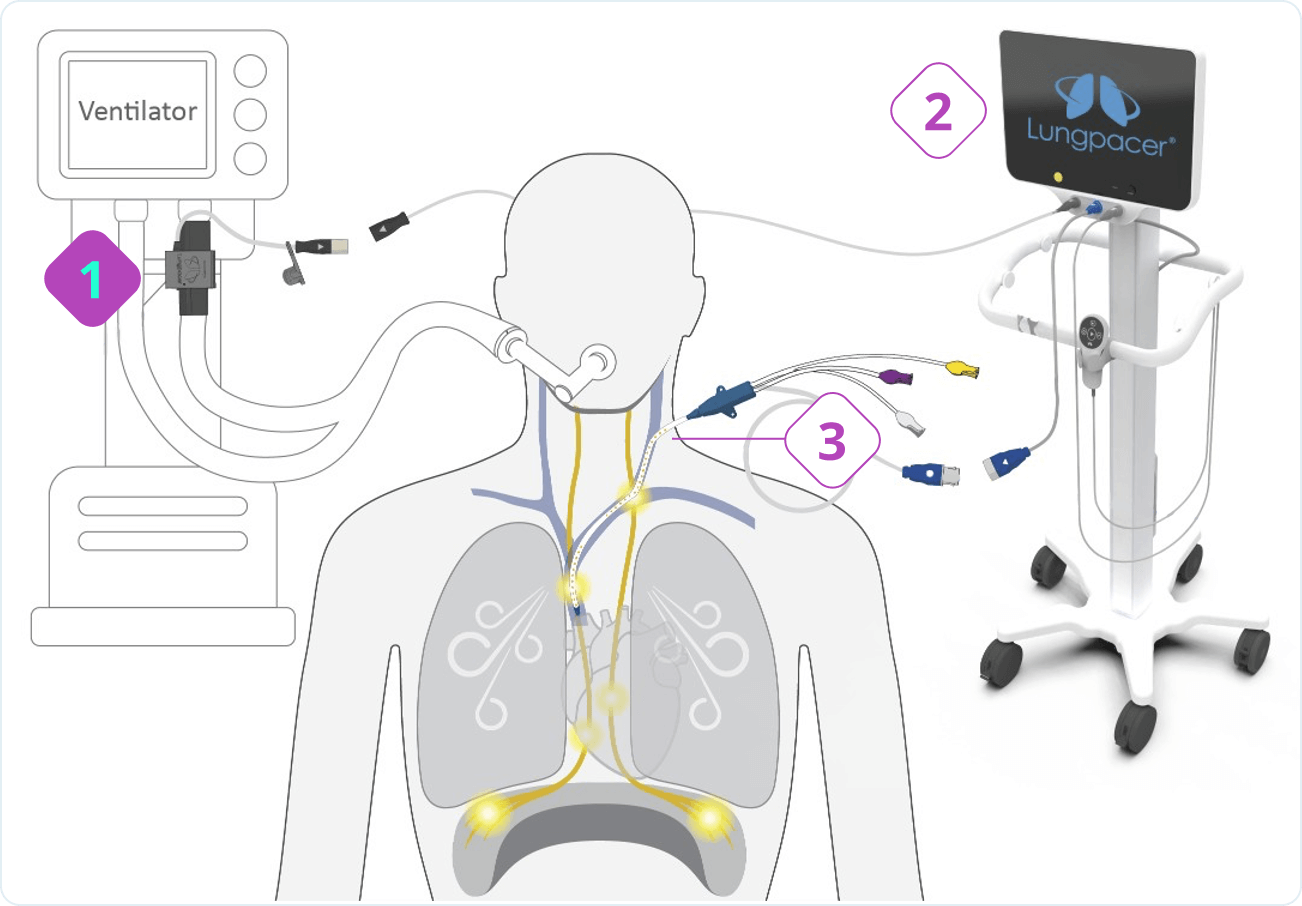
Diaphragm Dysfunction Develops Rapidly During Mechanical Ventilation (MV) and is a Principal Driver of Weaning Failure
Diaphragm muscle can atrophy 50% in only one day on mechanical ventilation1 and this dysfunction produces an 8x greater risk of weaning failure2. The weaning process is responsible for 40-50% of total mechanical ventilation time3.